Coronavirus: Singapore residents look to Sinovac shot as alternative to mRNA vaccines
- Alongside Singapore’s inoculation drive of Pfizer-BioNTech and Moderna shots, private doctors can apply to administer jabs from a bank of 200,000 Sinovac doses
- The WHO’s approval of the Chinese shot for emergency use gives a boost of confidence to people who cannot take mRNA jabs for medical reasons, although they have to bear all costs and risks involved
The 27-year-old IT worker, who only wished to be known by his surname, said he preferred its use of “traditional technology”, referring to how the Chinese-made jab uses a weakened or inactivated disease germ in the body – the same technology used in vaccines for polio and rabies.
On Monday, Health Minister Ong Ye Kung said private health care providers would be allowed to import vaccines not approved by Singapore regulators but fell under the WHO’s emergency-use listing. These include the Johnson & Johnson as well as the Oxford-AstraZeneca jabs, among others.
“As and when the WHO approves the Sinovac vaccine under its emergency-use list, the licensed health care institution can apply to [the Ministry of Health] to draw on our existing stock of 200,000 doses to administer to those who wish to have it,” Ong said.
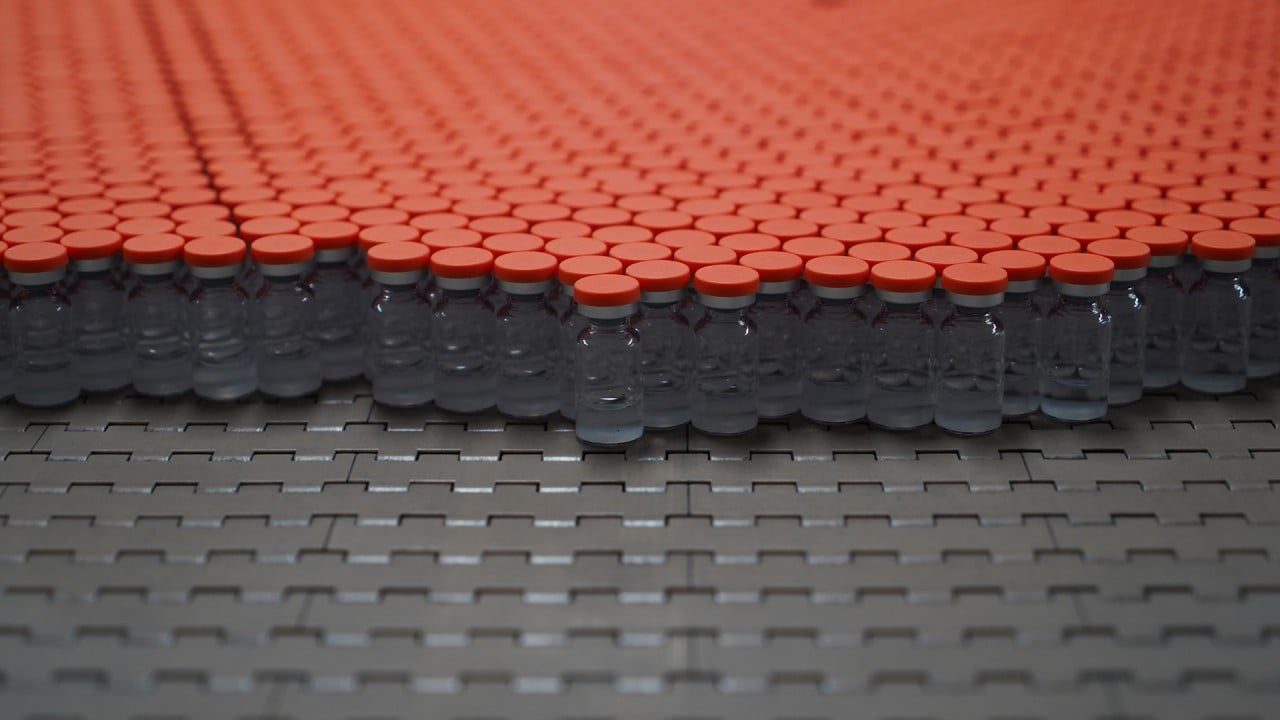
02:35
Inside a plant in China producing the WHO-approved Sinovac Covid-19 vaccine
Singapore’s authorities have said the Sinovac shot has a shelf life of two years. But in recent months, members of the Chinese community have expressed concerns online about the vaccines being left untouched, especially as Singapore sees a sharp uptick in domestic cases and officials say there are limited vaccine supplies.