US authorises fourth coronavirus shot for over 50s as BA.2 subvariant spreads
- The Food and Drug Administration says the decision is based on emerging evidence that an additional booster improves protection against severe Covid-19
- An Israeli study shows that three doses of current generation mRNA vaccines have hit a ceiling in terms of the immune response generated
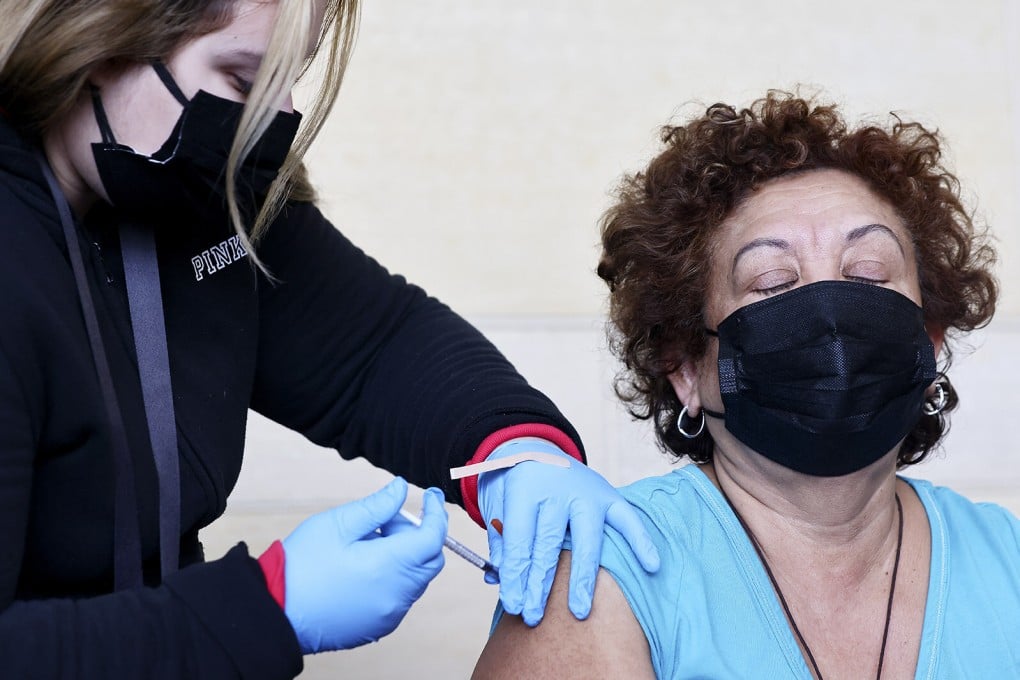
The United States on Tuesday authorised a fourth dose of either the Pfizer-BioNTech or Moderna Covid-19 vaccines for people 50 and older, as authorities warn of a possible new wave driven by the BA.2 subvariant.
The Food and Drug Administration (FDA) said in a statement it had based its decision on emerging evidence that an additional booster, given four months after the last, improved protection against severe Covid-19 and was not associated with new safety concerns.
Additionally, people with immune compromising conditions who have already received four shots, with their latest at least four months ago, are now eligible for a fifth dose.
That includes people living with certain organ transplants.
The Pfizer vaccine will be available to immune compromised people aged 12 and over, while the Moderna vaccine will be available to those 18 and up.