Moderna coronavirus vaccine could be ready by end of year, US says as drug maker begins final-stage trial
- Phase three study with 30,000 subjects is first such trial under Trump’s Operation Warp Speed programme
- Moderna could have tens of millions of doses ready when and if vaccine is deemed safe and effective
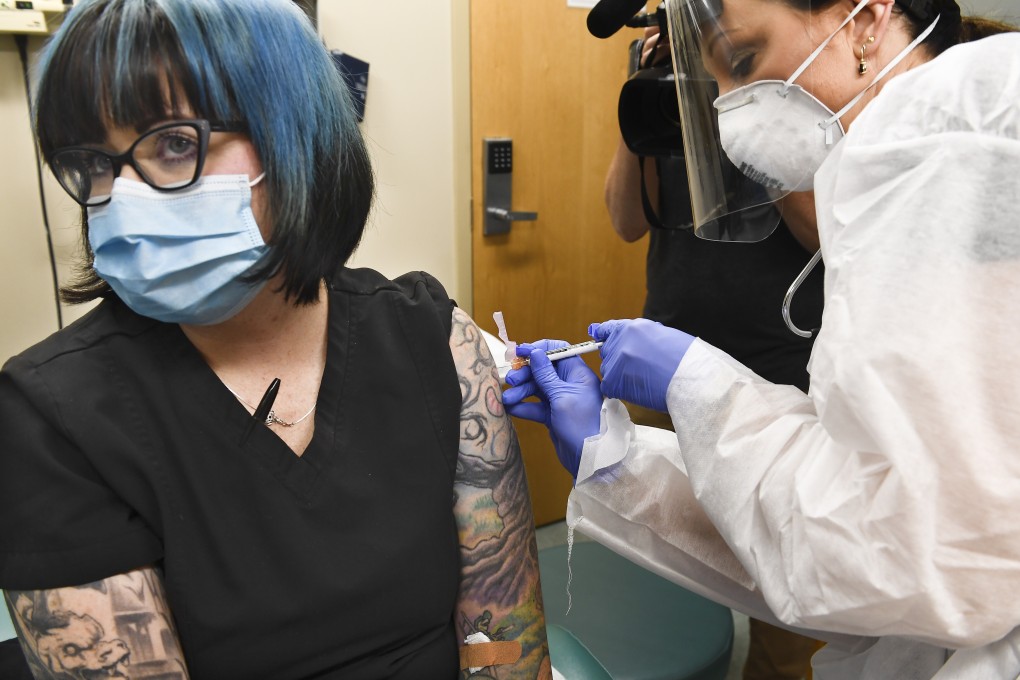
Moderna’s vaccine against Covid-19 could be rolled out by the end of this year, US officials said on Monday, after the drug maker announced the start of a 30,000-subject trial to demonstrate it is safe and effective, the final hurdle before approval by global regulators.
The trial is the first such late-stage study under the Trump administration’s programme to speed development of measures against the novel coronavirus, adding to hope that an effective vaccine will help end the pandemic. Shares of Cambridge, Massachusetts-based Moderna rose 9 per cent.
More than 150 coronavirus vaccine candidates are in various stages of development, with some two dozen prospects having advanced to human testing.
06:17
‘Robust immune responses’ found in Covid-19 vaccine clinical trials point to 2021 release
“Having a safe and effective vaccine distributed by the end of 2020 is a stretch goal, but it’s the right goal for the American people,” National Institutes of Health (NIH) Director Francis Collins said in a release announcing the start of the large phase three trial.
Manufacturers are ramping up production while testing is under way to respond as soon as possible to virus, which is still spreading rapidly around the world. Covid-19 has killed about 650,000 people worldwide and battered economies.