Advertisement
Chinese drug firm Sinopharm finally publishes Covid-19 vaccine trial data
- Interim phase 3 results suggest two products have efficacy above 70 per cent for symptomatic cases but there is little data on elderly and vulnerable groups
- More than 200 million doses have already been administered worldwide and a vaccine made by the firm’s Beijing subsidiary has been approved by the WHO
Reading Time:4 minutes
Why you can trust SCMP
77
The Chinese drug maker Sinopharm has finally published the interim results from a phase 3 trial of its Covid-19 vaccine after more than 200 million doses have been administered worldwide.
The study, published in the Journal of the American Medical Association on Wednesday, has finally answered scientists’ calls for greater transparency, but the results do not cover some of the most vulnerable groups.
Trial data shows a vaccine made by Sinopharm’s Beijing subsidiary, which has been approved by the World Health Organization for emergency use, could offer 78.1 per cent of protection against symptomatic Covid-19 but the rate dropped to 73.5 per cent after taking asymptomatic cases into account.
Another vaccine by the company’s Wuhan subsidiary, which is in the process of applying for WHO emergency use licensing and has been conditionally approved for general use in China, had 72.8 per cent efficacy against symptomatic cases and 64 cent including asymptomatic ones.
The results were based on data collected from 40,411 participants in the United Arab Emirates and Bahrain last year.
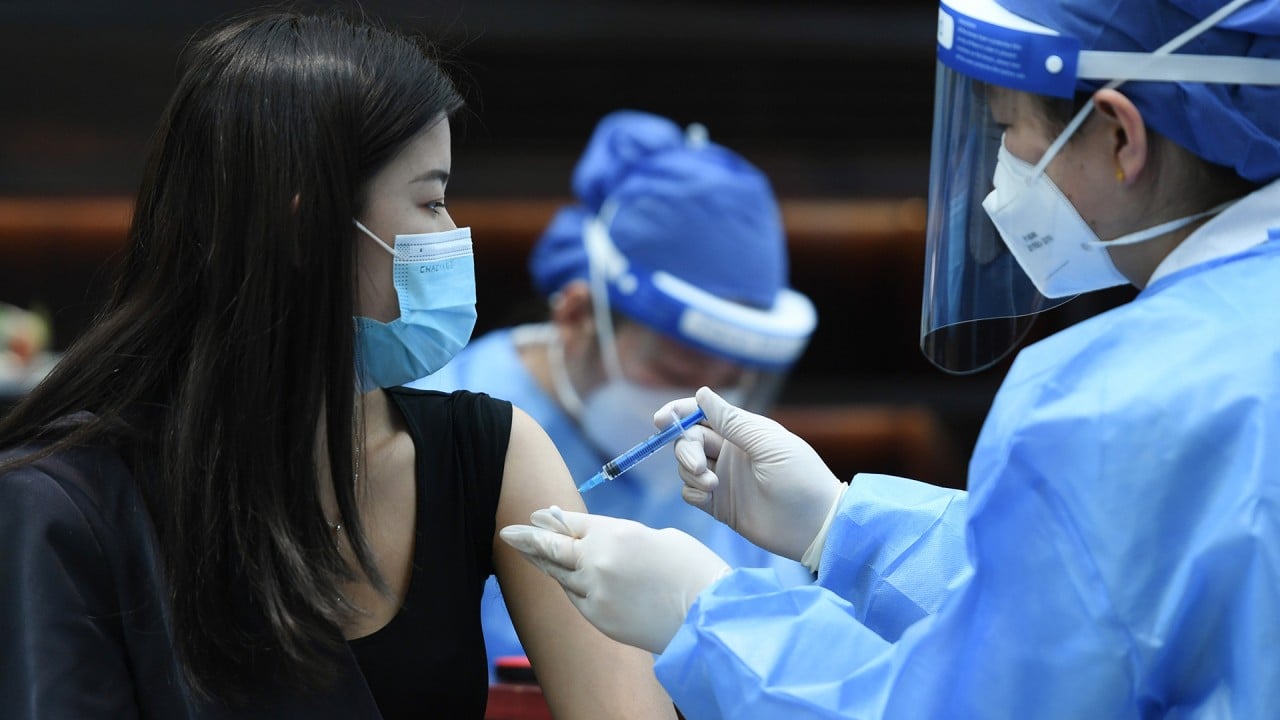
02:06
After ramping up vaccination drive with incentives, China administers 200 million Covid-19 shots
After ramping up vaccination drive with incentives, China administers 200 million Covid-19 shots
The trial participants, mostly healthy 18-59-year-olds, were equally divided into three groups inoculated with the vaccine from the Beijing subsidiary, the Wuhan subsidiary or a placebo.
Advertisement