Coronavirus: US starts clinical trial of Covid-19 vaccine that will last into next year
- The trial is taking place at a medical facility in Seattle, whose metropolitan area has seen 20 per cent of the total US cases
- The entire clinical trial process could take up to 14 months, the National Institutes of Health says
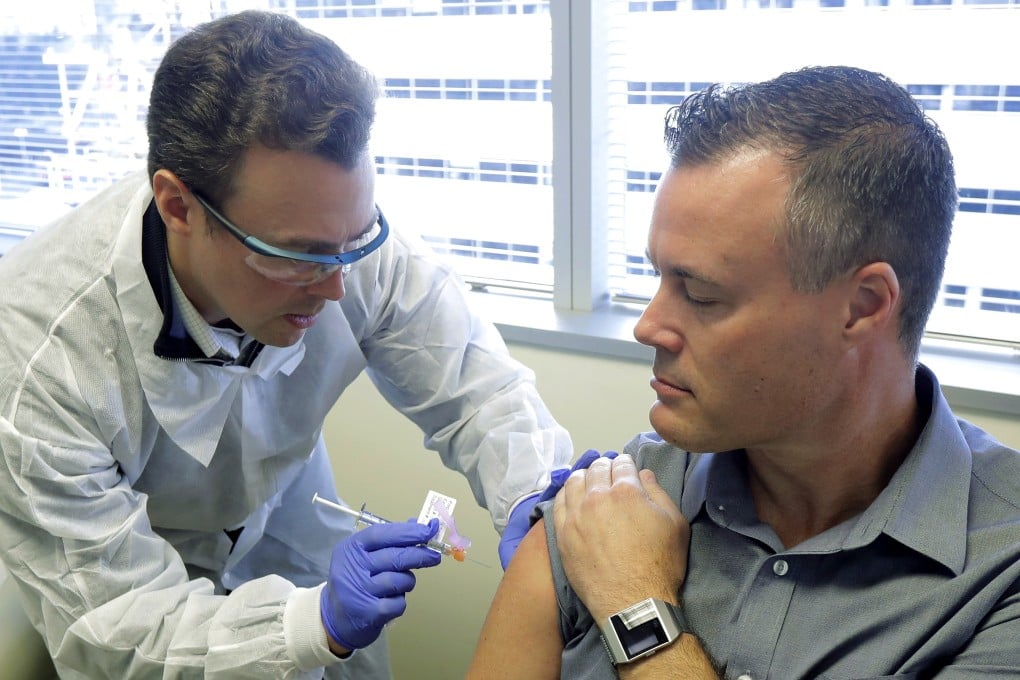
A clinical trial of an experimental vaccine against the virus that causes the Covid-19 disease began in the US on Monday, according to the National Institutes of Health (NIH), with the multistage process set to stretch into 2021.
The announcement by the NIH came as infections surged across the West and multiple countries announced full or partial shutdowns and curfew-like restrictions to curb the spread of the pandemic, which has sickened some 170,000 people and killed more than 6,500 people worldwide.
In a statement, the NIH said the first of 45 “healthy adult volunteers” received their first dose of the vaccine on Monday. The trial is being conducted in an open label format, meaning the volunteers – aged between 18 and 55 – are fully aware of its specifics.
Called mRNA-1273, the vaccine is jointly developed by scientists at the National Institute of Allergy and Infectious Diseases (NIAID) and the Massachusetts-based biotechnology company Moderna Inc.

The Washington-based CEPI global epidemic response coalition supported the development of the experimental vaccine, NIH said.