WHO approves India-made Novavax coronavirus vaccine for emergency use
- The decision is good news for poorer countries, as it paves the way for the jabs to be included in the UN-backed Covax programme
- The shots require only refrigerated storage – an appealing option for low-income countries compared to other vaccines that require very cold storage
The vaccine, known as Covavax, is the ninth to be granted an emergency use authorisation from the UN health agency, marking a vote of confidence for Novavax that could also mean the shots will be accepted by some countries that only admit travellers vaccinated with WHO-backed jabs.
The Serum Institute is producing the Novavax-developed vaccine and a big question is how much supply it can ship, and when.
The vaccine was long anticipated to help increase global vaccine supplies, as the shots require only refrigerated storage – an appealing option for low-income countries compared to other vaccines that require very cold storage.
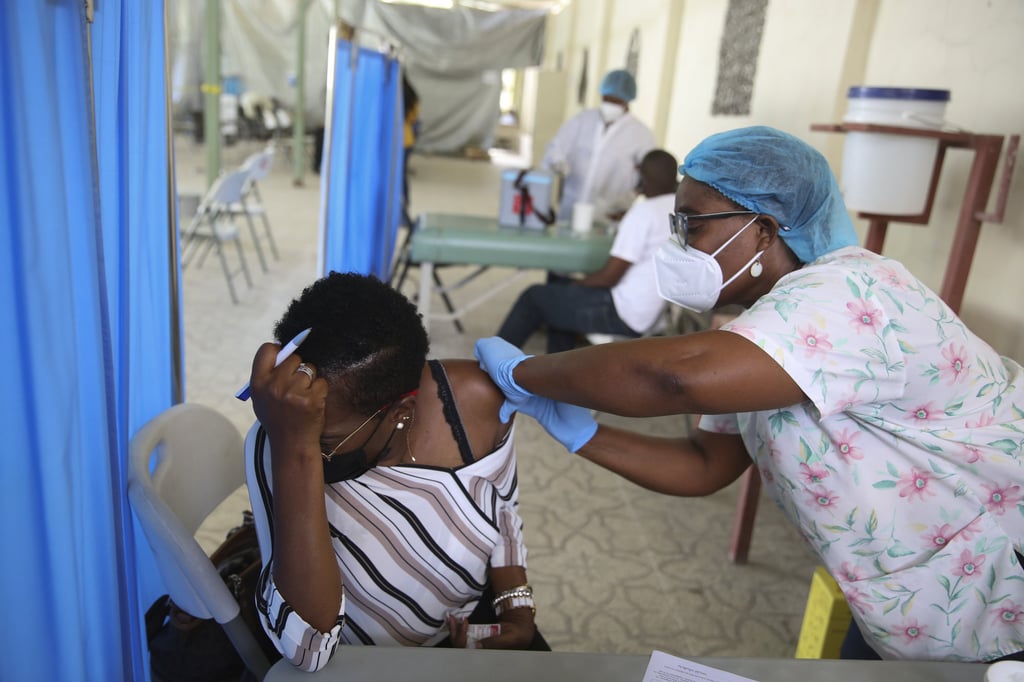
“This listing aims to increase access particularly in lower-income countries, 41 of which have still not been able to vaccinate 10 per cent of their populations, while 98 countries have not reached 40 per cent,” said Dr Mariangela Simao, WHO Assistant-Director General for access to medicines and health products, alluding to the vast inequity in access to vaccines between well-stocked rich countries and poorer ones that lack them.