Exclusive | Coronavirus: Bill Gates-backed Hong Kong start-up wins US FDA approval for its Covid-19 rapid test kit even amid US-China strain
- Hongkonger Ricky Chiu Yin-to founded Phase Scientific International in 2015 while pursuing a PhD at the University of California
- The biotech start-up is building a facility in California that will focus on developing liquid diagnostic tests for various cancers, and plans to build a similar one in Shenzhen

Phase Scientific International (PSI), the Hong Kong start-up that edged out established mainland Chinese peers to win US approval for a Covid-19 rapid antigen test (RAT) kit, owes its success to its US roots and experience in the field, according to its founder.
The Bill and Melinda Gates Foundation-backed biotech company last July became the first in the mainland China, Hong Kong, Macau and Taiwan region to win approval from the US Food and Drugs Administration for emergency use for its Covid-19 kit.
Ricky Chiu Yin-to, a Hongkonger who moved to the US to pursue a PhD in bioengineering at the University of California in Los Angeles, founded the company in 2015. He relocated its headquarters from California to Hong Kong in 2018.
“This move, a gamble at the time, has fully paid off,” Chiu who is also the CEO told the South China Morning Post. “The technology I was working on was good but was mature and very competitive in the US … hence the thought of coming back to Hong Kong and tapping opportunities in China.”
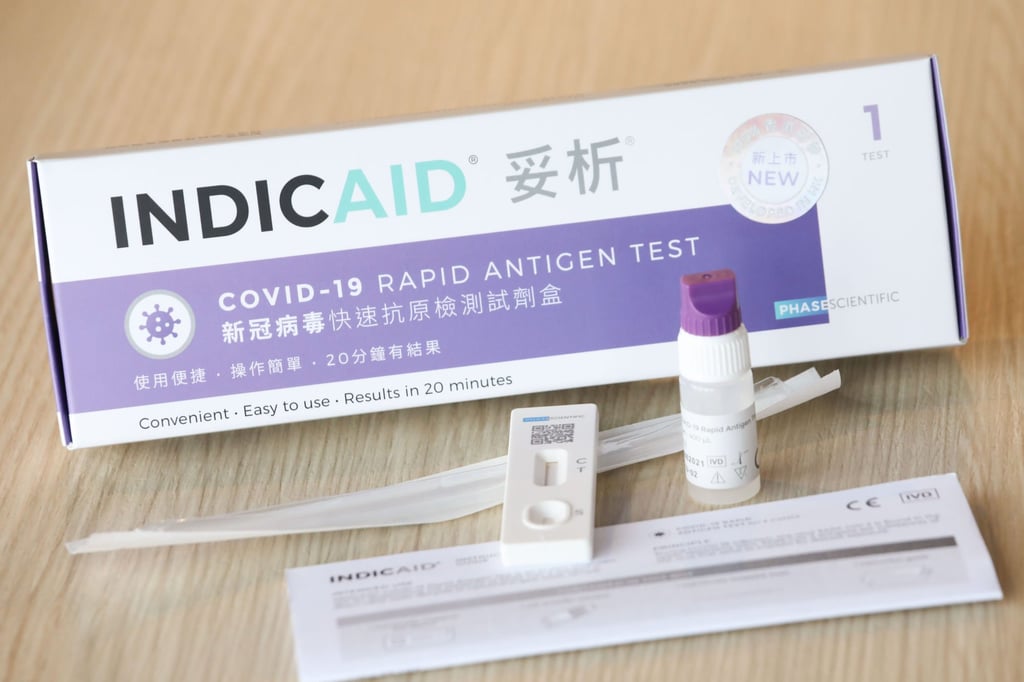
PSI initially focused on commercialising a patented methodology that improves the accuracy of liquid diagnostic tests by concentrating and purifying target molecules in blood or saliva. It started off by launching a US$10 tooth decay risk detection kit in late 2018.